Author:
Seppo Vahasalo, Product Line Manager, Medical Devices, SGS
Date
10/30/2012
Manufacturers are required by the 3rd edition of IEC 60601-1 to avert the poor usability risks involved in identification, marking, and documents, which can be achieved by an "application of usability engineering to medical devices" compliant with IEC 62366. Improving usability for safety and competitiveness The Institute of Medicine issued a report in 1999 entitled To Err is Human: Building A Safer Health System. The report contended that over 44,000 people die in US hospitals each year resulting from preventable medical errors, and perhaps as many as 98,000 people. The report stated most medical mistakes are not due to individual carelessness but rather to faulty processes, conditions, and systems that cause people to make errors or fail to avoid making them. The European MDD and United States FDA both demand that medical devices are developed by taking errors arising from risk-of-use and human factors into account. The IEC 60601-1 3rd edition and specifically IEC 60601-1-6, are examples of international standards that seek to minimize the risks presented by poor usability with the application of usability engineering. There is a responsibility, wherever medical devices are used, to make sure all products on the market are well-designed and ergonomic. The simpler a medical product is to use, the safer it will be for patients. Consequently, safer products increase their market competitiveness. Guidance and implementation of IEC 62366 IEC 62366 provides extensive guidance about how best to minimize the risks with usability engineering. The 100-page document that defines the standard presents a user-interfaced medical design cycle (figure 1) within the context of a regulated-product management cycle (figure 2).
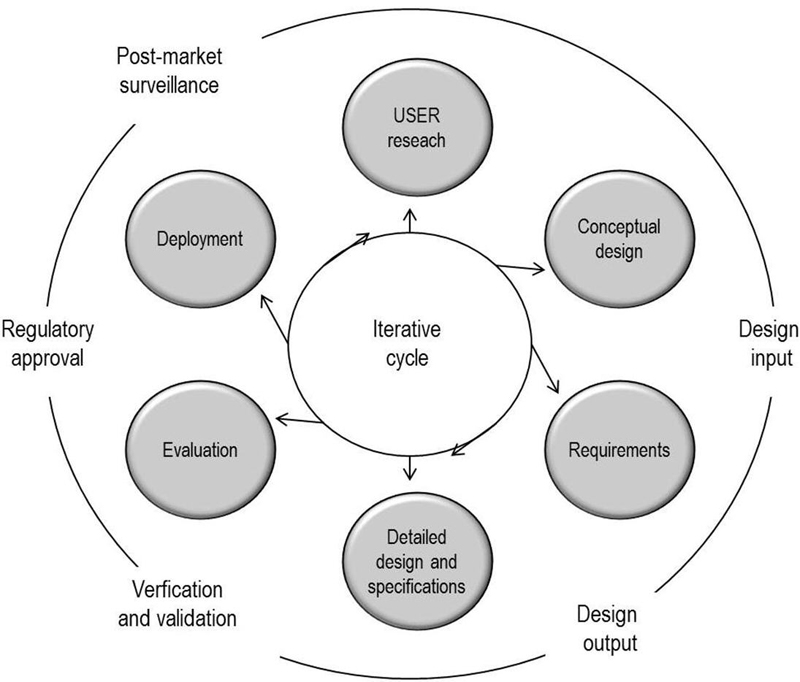
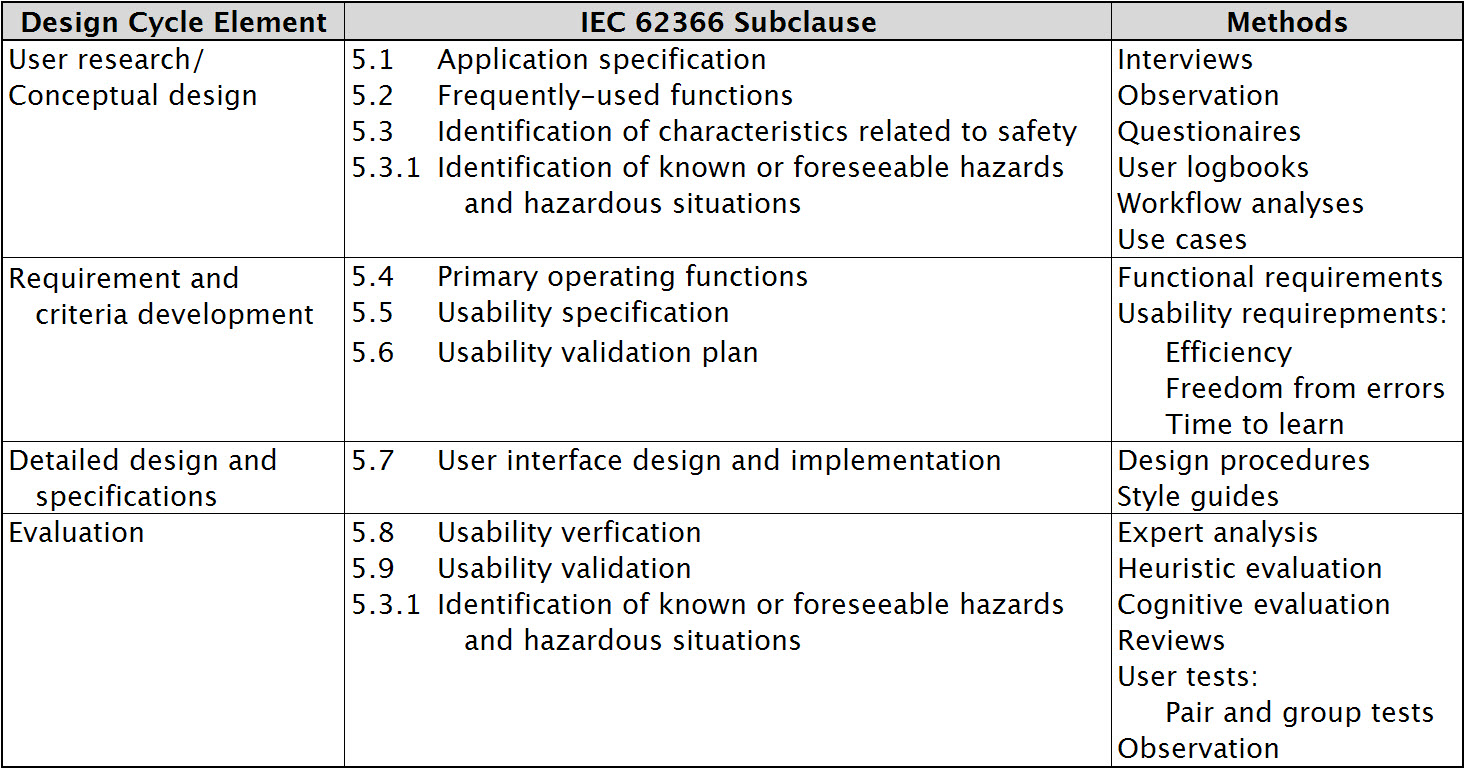
Table 1 further describes this usability-engineering process and provides an understanding of how IEC 62366 designates design-cycle steps. Updating existing products Many of the products already in use were not explicitly designed with a usability-engineering process. Proposal 62A/799/DC seeks to address this by initiating an IEC 62366 fast-track amendment to compliance. The proposed amendment introduces an evaluation of UOUP (user-interface of unknown provenance) and a new Annex K to ensure engineering processes include usability in legacy devices, minor revisions of devices, and standard components embedded in medical devices. Adding good usability to a product is not possible as an afterthought. Design teams should integrate usability engineering from the outset and it is necessary to document the process continuously to comply with IEC 62366. Although the regulations demand usability engineering, good usability can also serve as the basis for product differentiators that offer competitive advantages. Questioning and observing users to learn how they use various versions of equipment and early prototypes is an invaluable part of the process. When users know how to use a product intuitively and have no need to find workarounds, your product design is certainly going in the right direction. Designing an aesthetically pleasing medical device or software application also reaps dividends. SGS Group