Author:
Peter Blyth, Industry Director Medical, XP Power
Date
10/29/2011
The internationally accepted IEC 60601-1 standard has been continuously developed to help alleviate safety issues relating to all manner of medical equipment. Engineers need to be aware that when using these standards, there are a number of key dates specified for the implementation of the 3rd edition, and that these vary by region. In Europe, from 1st June 2012 the 2nd edition (EN60601-1/A2:1995) will be withdrawn, and all products will need to be certified to the 3rd edition, EN60601-1:2006. This includes both new products introduced to the market and products already on sale. The situation is rather different in the United States. The cessation date for 2nd edition (UL60601-1:2003 1st edition) is 30June 2013 but, unlike the EU, the FDA only requires that new products brought to market after this date will need to be 3rd edition certified (ANSI/AAMI ES60601-1:2005). In Canada the cessation date for 2nd edition (CAN/CSA C22.2 No. 601.1) is 1 June 2012, but again the 3rd edition (CSA?C22.2 NO. 60601?1:08) is only needed for products new to the market after this date.
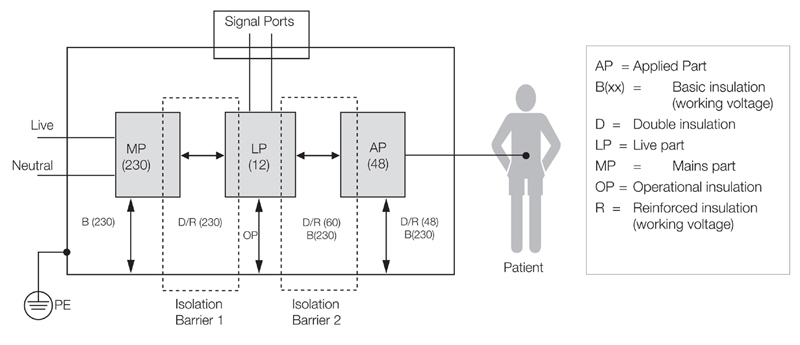
Another complicating factor is the particular standards that are part of the 60601 family. These are commonly referred to as "part 2's" and will have the standard number 60601-2-xx, such as IEC60601-2-46, particular requirements for the safety of operating tables. Where these are applicable the equipment needs to be certified to these standards and therefore the date for 3rd edition adaption will be dictated by the date that the 2nd edition part 2 is withdrawn. This could be before the main date or after it. Some countries are, to date, not adopting 3rd edition. This means that equipment will need to be certified to IEC60601-1/A2:1995 2nd edition for those regions, because after 1st June 2012 EN60601-1 2nd edition will be withdrawn and UL60601-1 will be withdrawn in June 2013. At XP Power all power supplies are certified to 3rd edition (with 2 x Means Of Patient Protection on the majority of power supply) but to also test against 2nd edition. The rationale here is that following 2 x MOPP in 3rd edition is equivalent to 2nd edition in terms of separation distance, insulation schemes and dielectric strength requirements. The OEM can claim the safety of the power supply is at least as good as the current standard (2nd edition) and will still maintain the equipment 2nd edition approval, even with a 3rd edition (2 x MOPP) approved PSU. (See clause 3.4 and clause 54 in UL60601-1:2003) One of the most significant changes in the 3rd edition is that equipment manufacturers must now follow a formal risk management procedure that follows the ISO 14971 model, which effectively means compliance with a process standard as well as the fundamental product standard. While the 2nd edition simply addressed basic safety issues to ensure freedom from any electrical, mechanical, radiation, and thermal hazards, it did not require devices to remain functional—fail-safe was adequate, and compliance with test criteria relied upon a pass/fail result that did not take into account the essential performance of the device-under-test. Recognizing these limitations, the 3rd edition introduces specifications for "essential performance" that requires equipment to continue functioning as its designers intended throughout the test process. Within the electrical safety arena, the standard continues to require that equipment implements two Means of Protection (MOP) such that if a failure occurs within one area, a second mechanism safeguards the operator and/or the patient against any electric shock hazard. Figure 1 models the insulation diagram that applies to the main circuit blocks in a notional medical device, and shows the two isolation barriers that provide the two Means of Protection that must be present within a device that may come into contact with a patient. The standard allows for three defensive approaches that may be used in various combinations—safety insulation, protective earth, and protection impedance. It's therefore essential to determine several key factors from the outset of the equipment design process, including its insulation class and whether it will rely upon a protective earth connection. These considerations extend to the "applied part", if present, that is deliberately attached to the patient. Such applied parts are separately classified as to the level of electric shock protection that they provide. Significantly for power supplies, the 3rd edition distinguishes between protecting the equipment's operator and the patient within its Means of Operator Protection (MOOP) and Means of Patient Protection (MOPP) categories. This distinction can result in quite different safety insulation and isolation requirements for circuits that operators and patients may come into contact with. Specifically, anything that falls within the remit of operator protection only has to meet the clearance and creepage requirements that IEC/EN 60950 specifies for general-purpose information and technology equipment. By contrast, circuitry that falls within the realm of patient protection must meet the far more exacting requirements of the 2nd edition of IEC 60601-1. As to who determines whether it is MOOP or MOPP is up to the manufacturer and they will need to record this in the risk management file. Choosing a power supply with only MOOP, other isolation schemes need to be in place between the output and the patient if the equipment is to come in contact with the patient. It complicates the design and increases cost - even though the cost of the MOOP power supply might be less than an MOPP power supply. No matter whether MOOP or MOPP is chosen the standard still requires that the leakage current requirements are met. For the power supply this means 300uA for the USA and 500uA for the EU. At XP Power, the power supply for a medical device must provide the highest degree of protection and reduce the risk of a shock hazard and have make our power supplies with 2 x MOPP from input to output (mains to low voltage dc). This gives customer flexibility and minimizes the risk of a shock hazard. A major component to the 3rd edition is for a Risk Management Process to be included as part of the submittal to the certified body who will undertake the product certification. While risk management is not a new concept to device manufacturers it is new for the power supply manufacturers. Under 2nd edition the certified body would test against a pass/fail criterion which was very black and white. It is true that this same pass/fail criterion exists under 3rd edition but it is also required that risk management is included. The IEC recently published a guidance note for power supply manufacturers stating that 3rd edition could be gained without risk management, but that the device manufacturer would have to cover this during their submittal, but this merely pushes the cost back to the device manufacturer and ultimately the device manufacturer will require the PSU manufacturer to provide risk analysis, Failure Mode Effects Analysis (FMEA) etc. If the power supply manufacturer doesn't have this prepared then there could be a delay in getting this valuable information. XP Power submits a risk management process with all submittals and make this available to customers where necessary. Customers can now assume the power supply is a "black box" and just consider the implications of output failing etc. The internal analysis is done by XP Power. In order to achieve this, we embraced ISO14971 such that risk management is now part of our design process. For the production aspects of certification we have our factory approved to ISO13485; this being the quality management system for medical devices. www.xppower.com