Author:
Peter Blyth, Medical Industry Director, XP Power
Date
10/01/2010
Safety is paramount wherever power supplies are used, but nowhere more so than in medical applications where even small parasitic leakage currents may compromise safety. First published in 1977, the internationally accepted IEC 60601-1 standard has been continuously developed to help alleviate safety issues relating to all manner of medical equipment. The latest version is the third edition that was published in December 2005 and that has been adopted on different timescales around the globe. In the European Union, the standard appeared as EN 60601-1:2006 and its three-year transition date expired last September. Similarly, most major countries have adopted IEC 60601-1 as their national standard, in some cases with national variations such as ANSI/AAMI ES60601-1 in the US and Canada's CAN/CSA C22.2 No. 601.1.
Clearly, minimizing risk is a crucial part of any medical equipment design process, and it is in strengthening this aspect that the updates within the third edition focus upon. The standard's range extends to equipment that has a single connection to the ac line supply and that is intended to diagnose, treat, or monitor patients under medical supervision. Qualifying equipment includes devices that make physical or electrical contact with the patient, and/or transfer energy to or from the patient, and/or detect energy transfer to or from the patient. The most significant change that the third edition introduces is that equipment manufacturers must now follow a formal risk management procedure that follows the ISO 14971 model, which effectively means that you now have to comply with a process standard as well as the fundamental product standard. While the second edition simply addressed basic safety issues to ensure freedom from any electrical, mechanical, radiation, and thermal hazards, it did not require devices to remain functional—fail-safe was adequate, and compliance with test criteria relied upon a pass/fail result that did not take into account the essential performance of the device-under-test. Recognizing these limitations, the third edition introduces specifications for "essential performance" that requires equipment to continue functioning as its designers intended throughout the test process. Within the electrical safety arena, the standard continues to require that equipment implements two Means of Protection (MOP) such that if a failure occurs within one area, a second mechanism safeguards the operator and/or the patient against any electric shock hazard. Figure 1 models the insulation diagram that applies to the main circuit blocks in a notional medical device, and shows the two isolation barriers that provide the two Means of Protection that must be present within a device that may come into contact with a patient.
The standard allows for three defensive approaches that may be used in various combinations—safety insulation, protective earth, and protection impedance. So far as insulation is concerned, a change in terminology sees basic insulation allocated a 1 MOP rating while double or reinforced insulation rates as 2 MOPs. It's therefore essential to determine several key factors from the outset of the equipment design process, including its insulation class and whether it will rely upon a protective earth connection. These considerations extend to the "applied part", if present, that is deliberately attached to the patient. Such applied parts are separately classified as to the level of electric shock protection that they provide. Significantly for power supplies, the third edition distinguishes between protecting the equipment's operator and the patient within its Means of Operator Protection (MOOP) and Means of Patient Protection (MOPP) categories. This distinction can result in quite different safety insulation and isolation requirements for circuits that operators and patients may come into contact with. Specifically, anything that falls within the remit of operator protection only has to meet the clearance and creepage requirements that IEC/EN 60950 specifies for general-purpose information and technology equipment. By contrast, circuitry that falls within the realm of patient protection must meet the far more exacting requirements that the second edition of IEC 60601-1 introduced. Furthermore, some equipment may include parts that fall under both categories, with risk analysis techniques often being used to determine the respective boundaries. In this respect, it's arguable that the third edition of IEC 60601-1 defines patient vicinity less well than in its predecessor. While the second edition drew a boundary of 1.5 m around the patient, the latest version employs risk assessment to determine where the patient is and the likelihood of him or her making contact with any part of the medical equipment. If the risk assessment shows that there is negligible likelihood of contact being made, it's theoretically possible to relax the insulation requirements to those of IEC/EN 60950. This also applies to conscious patients, as the standard now differentiates between conscious and unconscious states and makes the implicit assumption that those who are unconscious require a greater degree of protection. That is, in theory at least, the safety requirements for equipment that only comes into contact with conscious patients may be the same as the requirements for operators. Selecting suitable power supplies The main attraction of being able to specify a supply that meets normal IEC/EN 60950 standards is the potential for cost reduction. For instance, a supply that's built to meet IEC/EN 60601-1's far stricter demands to satisfy its Means of Patient Protection category requires significantly larger creepage distances and air clearances that normal commercial-off-the-shelf supplies employ, together with greater levels of dielectric breakdown test voltages. However, any supply that is used within a patient's vicinity still has to meet IEC/EN 60601-1's earth leakage current requirements, which would almost certainly require significant modifications to a normal commercial unit. Such modifications typically include reducing the values of the Y-capacitors that help reduce the earth leakage current but this has a negative effect on the emissions produced by the power supply. As a result, the modified unit is less likely to meet EMC regulations and may require additional internal and/or external filtering. Re-qualifying a modified supply for safety or EMC concerns can then be a costly and time-consuming exercise. Marketing considerations can play an important part here too. Despite the cost savings that using standard commercial supplies might present, many medical equipment manufacturers still choose to specify IEC/EN 60601-1-approved parts for any product that is likely to come into contact with a patient, as to do otherwise may compromise salability. From a commercial perspective, the manufacturer faces two main choices here—to possibly save money by purchasing IEC/EN 60950-compliant supplies when the risk assessment determines that this is an option, or to go for a cost-effective IEC/EN 60601-1 approved unit. In a parallel development, component technology and design technique improvements now enable power-supply manufacturers to offer units that simultaneously meet industrial, information technology, and medical standards, with volume manufacturing lowering costs to make medical-quality supplies cost-competitive with commercial units.
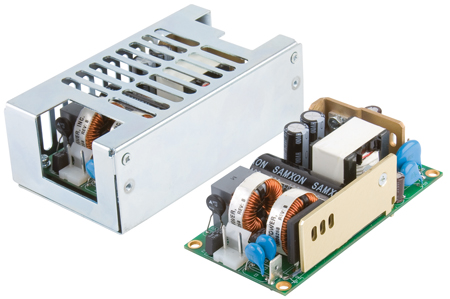
For instance, a typical 60W medically-qualified power supply costs around $35 in quantities of a few hundred pieces. Substituting a normal IEC/EN 60950-compliant part is unlikely to save more than $5, while at the same time limiting application flexibility. Worse, if you then have to modify the commercial-quality supply to meet say leakage current requirements, this choice is no longer lower cost. It may also limit your market, compromise your brand, or introduce additional and avoidable risks. As a result, specifying IEC/EN 60601-1 approved units that comply with Means Of Patient Protection (MOPP) is becoming a preferred approach for device manufacturers. Whilst the 3rd edition appears to offer the device manufacturer more options on the choice of power supply, the fundamental question of risk vs. cost must be considered; does one opt for a cheaper power supply with lower performance to save a few dollars or go for a higher specification power supply that might cost more but reduces the risk to as low as possible. After all, if you get it wrong in medical device design it could severely delay gaining regulatory approval or worse. 45W power supply with medical and industrial safety approvals XP Power has announced what is believed to be the world's smallest open frame 45W AC-DC power supply. Setting a new benchmark at this power level, measuring just 50.7 x 76.2 x 26.7mm (2 x 3 x 1.05 inches), the ECS45 single output power supply is 25% smaller than the current industry standard of 2 x 4 inches.

All models have a no load power of less than 0.3W, helping the end equipment comply with internationally recognized energy efficiency standards. In addition, these convection cooled units are highly efficient, typically 87%, resulting in less waste heat to dissipate. The lower profile ECS25 model provides 25W output within the same footprint. They can provide full power output up to + 50 degrees C without the need for any external fans or forced airflow and operate up to + 70 degrees C with derating. Both models provide the nominal outputs of +12, +15, + 24 or +48VDC. The ECS45 is also available with a single +5VDC 6A output. They have a wide input voltage range of 80-264VAC and are approved for Class I and Class II applications. The units meet UL60601-1 / EN60601-1 medical equipment safety standards and UL60950-1 / EN60950-1 standards for IT and industrial equipment. They also comply with the EN55011 / EN55022 level B standard for conducted emissions without the need for additional filtering components. Overvoltage, overload and short-circuit protection features are included as standard across the whole range. These power supplies suit designers of medical, IT or industrial equipment. Applications can cover a wide variety, such as broadcast equipment, computing and data storage. The medical safety approval makes it suitable for products such as portable medical devices, home healthcare devices and personal drug delivery equipment. Covered versions of each model are also available for Class I installations. www.xppower.com