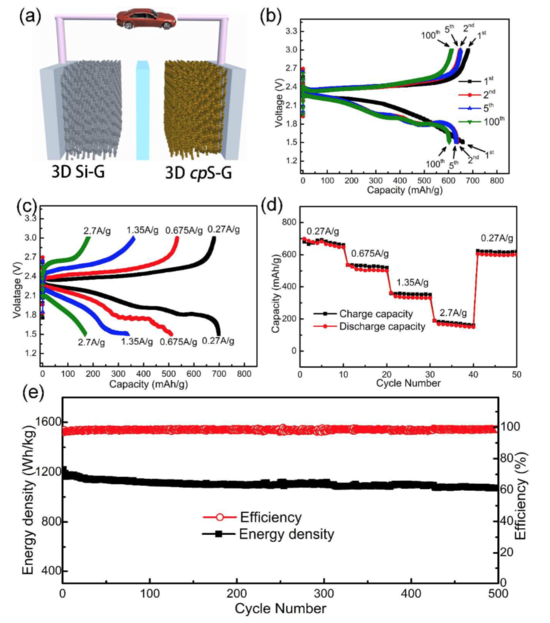
Electrochemical performances of full lithiated silicon-sulfur battery built from 3D cpS-G networks cathode and 3D lithiated Si-G anode. a) Schematic illustration of a new configured lithiated silicon-sulfur battery onto 3D graphene networks. b) Selected voltage profiles of lithiated silicon-sulfur battery from 1st to 100th at 0.27 A g-1 based on the total mass of cathode and anode). c) Selected voltage profiles of lithiated silicon-sulfur battery at various current densities from 0.27 to 2.7 A g-1. d) Rate capabilities of Si-S battery and e) long cycle performances of full lithiated silicon-sulfur battery built on 3D graphene
Researchers at Beihang University in Beijing have developed a new Li-sulfur battery using honeycomb-like sulfur copolymer uniformly distributed onto 3D graphene (3D cpS-G) networks for a cathode material and a 3D lithiated Si-G network as anode.
In a paper published in the RSC journal Energy & Environmental Science, they reported that the full cell exhibits superior electrochemical performances in term of a high reversible capacity of 620 mAh g-1, ultrahigh energy density of 1147 Wh kg−1 (based on the total mass of cathode and anode), good high-rate capability and excellent cycle performance over 500 cycles (0.028% capacity loss per cycle).
Lithium-sulfur (Li-S) batteries are of great interest as next-generation energy storage solutions, especially for electric vehicles, due to their high energy density, low production cost and environmental friendliness.
A number of challenges—in both sulfur cathode and lithium-metal anode—are retarding their commercialization, however. On the cathode side, the inherent insulation of sulfur (5×10-30 S cm-1) and high solubility of polysulfide intermediates commonly cause large active-material loss and poor cycle performance.
A number of approaches have been taken to address these cathode issues, including embedding sulfur into various porous carbons such as activated carbons, macroporous, mesoporous and microporous carbons, generating sulfur-carbon hybrids with well-designed nanostructures.
While this has improved overall electric conductivity and inhibited loss of active materials, restricted pore volumes still limit the contents of sulfur and polysulfides.
Another cathode strategy has involved the use of sulfur copolymer; this has shown good inhibition of polysulfide dissolution, but needs improved electric conductivity.
On the anode side, the lithium-metal anode reacts with the commonly used organic electrolytes forms lithium dendrites during cycling, resulting in short life and sever safety issues.
Alloy-type anodes are potential alternatives because of similar voltage plateaus to lithium. A number of approaches have been proposed, including the use of Si nanowires and lithiated Si/SiOx nanospheres.
Read more at Green Car Congress