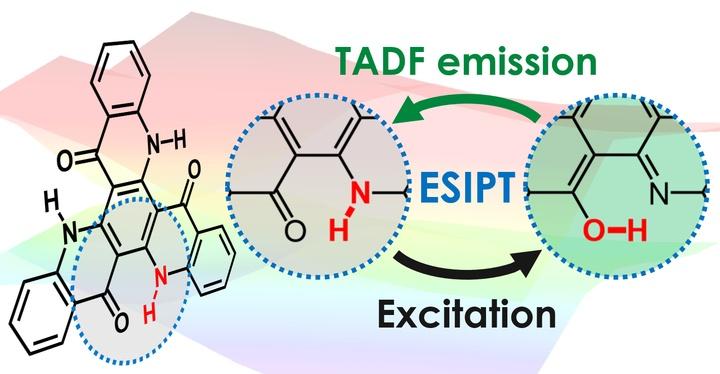
Excited-state intramolecular proton transfer (ESIPT) makes possible organic light-emitting diodes (OLEDs) that are highly efficient by creating the necessary conditions to enable thermally activated delayed fluorescence (TADF). After excitation of the emitting molecule, a hydrogen atom -- technically, just its nucleus -- is transferred to a different atom in the same molecule through a process called ESIPT. The reconfigured molecule can then undergo TADF to convert a high fraction of the excitations into light. Following emission, the molecule returns to its original state. This mechanism increases the molecular design strategies available for the creation of novel and improved light-emitting materials.
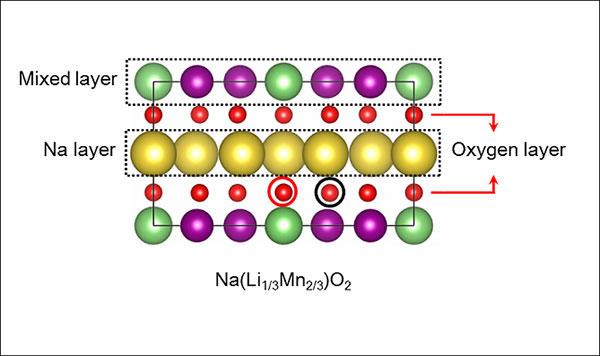
Researchers at the University of Texas at Dallas and Seoul National University have designed a novel battery cathode material that offers a potentially lower-cost, more eco-friendly option to lithium-ion batteries. Their sodium-ion design, which retains the high energy density of a lithium-ion cathode, replaces the most of the lithium atoms (green) with sodium (yellow). The layered structure of the new material also incorporates manganese (purple) and oxygen (red). The research is published in the journal Advanced Materials.